Abstract
Background
Immunotherapy development in pediatric AML is lagging because of dearth of validated AML-specific targets. We showed recently that mesothelin (MSLN) is highly expressed on the leukemia cell surface in a subset of pediatric AML patients, and validated MSLN as a therapeutic target using antibody-drug conjugates directed against MSLN (Kaeding et al., Blood Adv, 5:2350-2361, 2021). Antibody single-chain variable region (scFv) sequences derived from amatuximab recognizing MSLN and from either blinatumomab or AMG330 targeting CD3 were used to engineer and express two MSLN/CD3-targeting BsAbs: MSLN AMA-CD3 L2K and MSLN AMA-CD3 AMG respectively. Both these antibodies demonstrated anti-leukemic activity in mice engrafted with MV4;11 cells engineered to overexpress MSLN, while they failed to show any effect in mice bearing MV4;11 cells without MSLN, confirming that these antibodies specifically targeted MSLN (Gopalakrishnapillai et al., Blood, 134:3925, 2019).
Methods
MSLN cell surface expression was quantitated using BD Quantibrite PE Phycoreythrin Fluorescence Quantitation kit. 3x10 6 NTPL-146 cells were injected in NSG-B2m mice and 2x10 6 DF-5 cells were injected in NSG-SGM3 mice via the tail vein. Mice were randomly assigned to treatment groups when human cells were detectable in blood. The percentage of human chimerism in mouse peripheral blood was evaluated weekly by flow cytometry. Bipsecific antibodies were administered ip at 3 mg/Kg daily for six days. Human peripheral blood pan T cells from StemCell Technologies were injected iv (3x10 6 cells per mouse) to act as effector cells. Chemotherapy (DA) consisted of 3 doses of 1.5 mg/kg daunorubicin iv and 5 doses of 50 mg/kg cytarabine ip. Mice were monitored daily and euthanized when any of the experimental endpoints were met.
Results
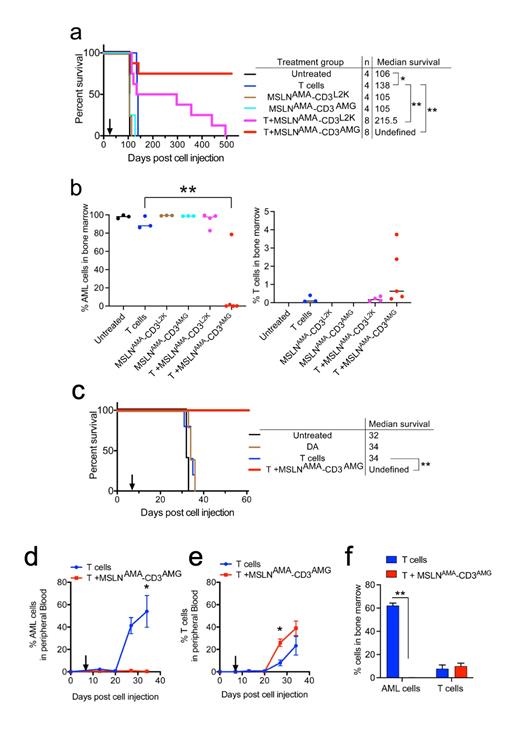
In this study, we evaluated the efficacy of two bispecific antibodies in two distinct patient-derived xenograft models of pediatric AML with endogenous MSLN expression quantitated at 6617 and 7414 MSLN antibodies bound per cell in NTPL-146 and DF-5 respectively. A Kaplan-Meier survival plot based on the time when each mouse reached experimental endpoint showed that 6/8 NTPL-146 engrafted mice receiving MSLN AMA-CD3 AMG and T cells survived disease-free until the end of the experiment at day 520 whereas all the mice in control groups had died by day 138 (Fig. 1a). The AML bone marrow load of MSLN AMA-CD3 AMG-treated mice was < 0.01% at 520 days, whereas the bone marrow load of mice from the other treatment groups was greater than 90% at the time of death, consistent with marrow failure as the proximal cause of death (Fig. 1b). These data show that treatment with MSLN AMA-CD3 AMG is curative in the vast majority of mice.
Treatment with MSLN AMA-CD3 L2K and T cells increased the median survival by 109.5 days compared to untreated mice while treatment with MSLN AMA-CD3 AMG showed a complete remission in 6/8 mice (**P<0.001). Thus, the survival benefit of both MSLN AMA-CD3 L2K and MSLN AMA-CD3 AMG treated mice greatly exceeded the allogeneic effect T cells alone, which only showed a 22-day improvement in median survival. Either MSLN AMA-CD3 L2K or MSLN AMA-CD3 AMG treatment alone showed no improvement in survival compared to untreated mice.
The efficacy of the potent BsAb MSLN AMA-CD3 AMG in comparison with chemotherapy (DA) was evaluated in DF-5. DA treatment, like T cell infusion, did not significantly change median survival compared to untreated mice, while BsAb MSLN AMA-CD3 AMG in the presence of human T cells was curative (Fig. 1c, **P<0.005). Mice treated with only T cells showed a rapid rise in AML cell percentage in peripheral blood until day 34 when they succumbed to disease, whereas the mice receiving T cells with BsAb had AML cell burden of less than 1% (Fig. 1d, *P<0.05). Mice treated with BsAbs showed greater expansion of allogeneic human T cells compared to mice receiving T cells alone (Fig. 1e, *P<0.05). Eight weeks post treatment initiation, the surviving mice were euthanized and AML burden in the bone marrow was evaluated. In contrast with mice receiving T cells alone, mice treated with T cells and BsAbs had no detectable AML cells (Fig. 1f, **P<0.005).
Conclusion
These data validate the efficacy of MSLN-targeting BsAbs in PDX models with endogenous MSLN expression. Because prior MSLN-directed therapies appeared safe in humans, MSLN-targeting BsAbs could be ideal immunotherapies for MSLN-positive pediatric AML patients.
Gopalakrishnapillai: Geron: Research Funding. Correnti: Link Immunotherapeutics: Current Employment. Kaeding: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Barwe: Prelude Therapeutics: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal